What are the units of rate constant for third order reaction. The concentrations of all three species are.
Units Of Rate Constants Qs Study
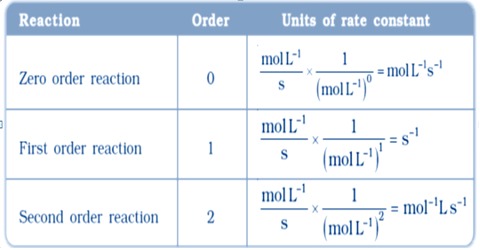
Unit of rate constant k mol L-1s-1.

. Characteristics of third order reaction 1 Unit of K-K 1 2t x 2a-x a 2a x 2 unit of K 1 time mole litre-1 mole litre-1 mole litre-1 2 x mole litre-12. Mol L-11-n s-1 mol L-11-3 s-1 mol-2 L2 s-1 Hence the value of X is 2. 2 The unit of velocity constant depends upon the units of concentration because.
Answered Nov 10 2021 by JohnAgrawal 909k points selected Nov 10 2021 by VijayThakur Best answer Correct Answer - d Unit of rate constant for a nth n t h order reaction is lit moln 1 sec1 lit mol n - 1 sec - 1 When n 3 the unit of rate constant is lit mol2 sec1 lit mol 2 sec - 1 or mol lit 2 sec1 mol lit - 2 sec - 1. Since A is assumed the only reactant for simplicity its order IS the reaction order. Because this equation has the form y mx b a plot of the inverse of A as a function of time yields a straight line.
The rate refers to the rate of the reaction. 5 rows More generally speaking the units for the rate constant for a reaction of order m n m. QUESTION 10 Which of the following would be a reasonable unit for the rate constant of a third order reaction.
USE WORDS TO FILL IN THE BLANKS NOT. Unit of rate constant of nth thorder reaction. The unit of the rate constant in a zero-order reaction is given by concentrationtime or Ms where M is the molarity and s refers to one second.
Step by step solution by experts to help you in doubt clearance scoring. Find the rate constant unit for the reaction 2NOO 2 2NO 2 1 Mark Ans. The rate constant for the reaction can be determined from the slope of the line which is equal to k.
In this reaction the rate will be written as. The rate of the reaction 2NOO 2 2NO 2 is given by RatekNO 2 O 2 Since it is a third-order reaction the unit of the rate constant is mol-2 s-1. Unit of K litre 2 mole -2 time-2.
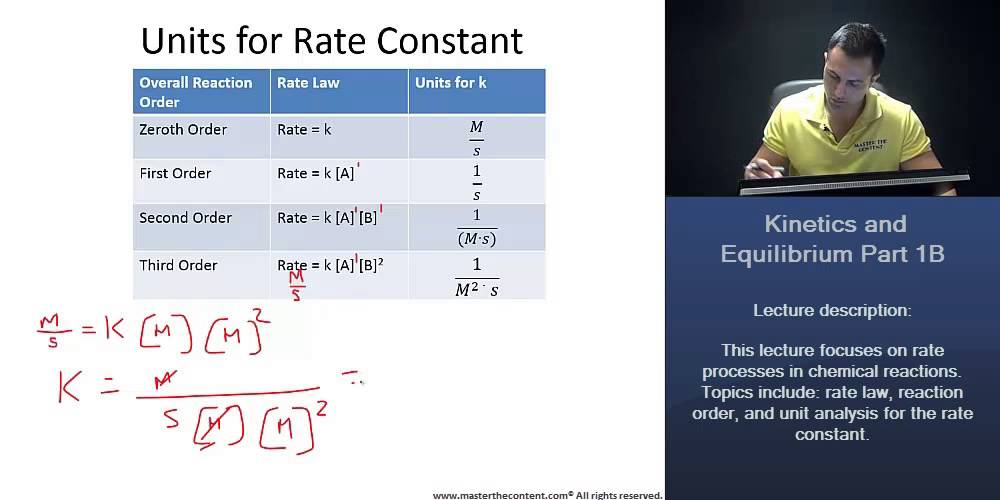
You will need to be able to write a general rate law and solve for the units. For a third order reaction the rate constant has units of liter squared per mole squares per second L 2 mol 2 s 1 or M 2 s 1 Other Calculations and Simulations For higher order reactions or for dynamic chemical reactions chemists apply a variety of molecular dynamics simulations using computer software. R a t e d A d t k A 0 k.
The unit of rate and rate constant are s Question The unit of rate and rate constant are same for a. The integrated rate law for the second-order reaction A products is 1 A_t kt 1 A_0. Different Cases in 3rd Order Kinetics.
Hello dosto In this video tutorial we provides you an unit of chemical constant of chemical kineticsit explains the rate law and order of reaction in chem. Join the 2 Crores Student community now. Rate k A Mt k M.
Rate k A 2 rate k A B Mt k M 2. The Rate Constant of Third Order Irreversible Reaction with Two Equal Reactant Concentrations formula is defined as the proportionality constant in the equation that expresses the relationship between the rate of chemical reaction and the concentrations of the reacting substances and is represented as k r C A C B2 or Third Order Reaction Rate Constant Reaction Rate. M -1 s -1 M -1 min -1 M -1 hr -1 etc.
If rate constant is numerically the same for three reaction of first second and third order respectively then which of the following is correct. If the units of time are s and of concentration are M then. Unit of rate is given by R mol Ls mol L-1 s-1 mol L-1 s-1 k mol L-1 3 k L 2 mol-2 s-1.
Rt kAn rt is the initial rate as a function of time t k is the rate constant A is the concentration of A and n is the order of A. To keep watching this video solution for FREE Download our App. Watch Video in App.
Measured in Square Cubic Meter per square Mole per Second Reactant A Concentration - Reactant A Concentration refers to the amount of reactant A present in the solvent at any given. K is the rate constant of the reaction. And for order three the rate constant has units of L 2 mol 2 s 1 or M 2 s 1 Plasma and gases Calculation of rate constants of the processes of generation and relaxation of electronically and vibrationally excited particles are of significant importance.
L2mol2 sec QUESTION 11 Given the data below for the reaction 2A 20-4CDE35 the reaction is order in A order in order in Cand order overall. S -1 min -1 hr -1 etc. R k A B Where k is known as the rate constant if the concentrations of both the reactants were one then the Rk which means that it wont depend on the initial concentration of the reactants.
Third Order Reaction Rate Constant - The Third Order Reaction Rate Constant is defined as the average rate of the reaction per concentration of the reactant having power raised to 3. Ms cdot. If we take a chemical reaction such as a A b B c C d D Then R k A p B q.
This browser does not support the video element. Three different cases may occur in the third-order reaction. Asked Jun 18 2020 in Chemistry by Prishabasu 961k points.
A Zero order reaction B First order reaction C Second order reaction D Third order reaction Easy Solution Verified by Toppr Correct option is A For a zero order reaction ratekA 0k Unitsmol L 1time 1.
Mcat Units For Rate Constant Zeroth Order First Order Second Order Third Order Youtube

Solved In A Second Order Reaction The Units Of Rate Constant Are N

Units Of Rate Constant Zero Order First Order Second Order Third Order Nth Order Reaction Youtube
0 Comments